🔍 Introduction to the Chemical Equation
Let’s begin our journey into the fascinating world of chemistry with a simple-looking but layered reaction:
What does it mean? Is it a riddle from an NYT crossword? Or is it a doorway to understanding organic chemistry in a more human, intuitive way?
This is more than just a combination of symbols—it’s a story of transformation, attraction, and synthesis. Let’s decode it.
🧪 Understanding the Reactants
💥 What is HCOOH (Formic Acid)?
Formic acid, chemically known as methanoic acid, is the simplest carboxylic acid. It’s found in the sting of ants and has the formula HCOOH. With both a hydrogen atom and a carboxyl group, it’s reactive and versatile.
🌿 What is CH₂=CH₂ (Ethene)?
Ethene, also called ethylene, is a gaseous hydrocarbon with a double bond between carbon atoms. This double bond is like an invitation—it loves to open up and bond with other atoms.
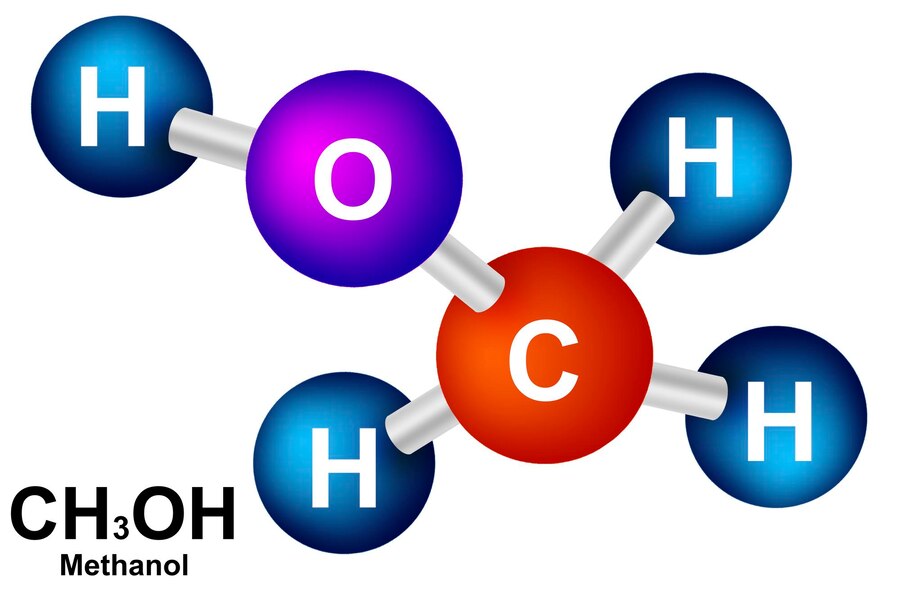
💧 The Role of H₂O in Organic Reactions
Water might seem like a passive bystander, but it often plays a pivotal role. In this case, it’s not just a solvent—it’s also a reactant, helping the other two get closer.
⚙️ The Type of Reaction Involved
Electrophilic Addition Explained
When ethene reacts, it tends to undergo electrophilic addition, where an electrophile (electron-deficient species) attacks the double bond.
Why Formic Acid Reacts with Alkenes
Formic acid can donate protons (H⁺), making it an ideal candidate to protonate alkenes and initiate the chain reaction.
Water as a Medium and Reagent
Water steps in as a nucleophile, attracted to positively charged intermediate molecules, completing the formation of the product.
🔍 Step-by-Step Breakdown of the Reaction
Phase 1 – Activation of the Alkene
The double bond in ethene gets protonated by the acidic hydrogen from formic acid. This opens the door for more bonding.
Phase 2 – Nucleophilic Attack
The now carbocationic ethene eagerly welcomes the hydroxyl group (from water) to stabilize itself.
Phase 3 – Formation of an Addition Product
The final product formed is 2-hydroxyethyl formate, a beautiful blend of acid, alkene, and water.
🧬 Mechanism: A Closer Look
Protonation of the Alkene
Ethene reacts with H⁺ from formic acid → forms a carbocation.
Intermediate Carbocation
This intermediate is very reactive, ready to bond with OH⁻ or H₂O.
Final Product: Hydroxyethyl Formate
We now have HO–CH₂–CH₂–O–CHO, a compound known for being both polar and reactive.
🏭 Industrial and Laboratory Applications
Use of HCOOH in Organic Synthesis
Formic acid is widely used in:
- Pharmaceuticals
- Dyes
- Rubber manufacturing
How Ethene Is Industrially Hydrated
Industries add water to ethene to make ethanol—a process similar to our reaction!
Water as a Reactant in Chemical Engineering
In hydration reactions, water is more than a solvent—it changes everything.
💞 Real-Life Analogy
A Love Story of Molecules – How They Connect
Imagine ethene as a bachelor with open arms (double bonds), and formic acid as someone offering a heart (H⁺). Water plays the role of the matchmaker, gently bringing them together.
The Matchmaker: Water’s Role in the Equation
Without water, ethene might just sit lonely. With water, it’s chemistry—literally!
🔥 Energy Considerations
Endothermic vs Exothermic
This reaction can be mildly exothermic, releasing energy when the new bonds form.
Activation Energy and Catalysts
While not always needed, acidic catalysts like H₂SO₄ can speed up the process.
🌍 Environmental and Safety Aspects
Formic Acid Handling Precautions
- Corrosive
- Eye and skin irritant
- Needs proper gloves and goggles
Ethene – A Flammable Friend
- Highly flammable
- Must be handled with care
Water – Universal Solvent but Not So Innocent
Though safe, water can participate in exothermic reactions that need moderation.
🎯 Conclusion
So there you have it—a seemingly simple chemical equation that unfolds into a narrative of reactivity, attraction, and creation. When HCOOH, CH₂=CH₂, and H₂O come together, we don’t just get a compound. We witness a reaction full of purpose, underlying much of organic chemistry’s elegance.
In understanding this, we see not just atoms and molecules, but dramas of the microscopic world, where every bond tells a story.
❓ FAQs
1. What does the reaction HCOOH + CH₂=CH₂ + H₂O form?
It forms hydroxyethyl formate, an ester formed via electrophilic addition and hydration.
2. Is this reaction reversible?
Under some conditions, especially with heat or catalysts, the reaction can be partially reversible.
3. Can it happen in living organisms?
Not exactly this way, but similar hydration and esterification reactions are common in biochemistry.
4. What is hydroxyethyl formate used for?
It’s used in solvents, resins, and sometimes in fragrance manufacturing.